News
News items will be published here, but also sign up to our Newsletters — see the form on the right.
You can receive updates from the News feed, either to your RSS reader or via email.
More misleading claims at NHS Homeopathic Hospitals
The adjudications by the Advertising Standards Authority about claims made by homeopaths and the Royal London Hospital for Integrated Medicine have all been published, so why were the Glasgow and Bristol Homeopathic Hospitals still making questionable claims?
 We won ASA complaints last year over claims made by the Royal London Hospital for Integrated Medicine (RLHIM) about Medical and Clinical Hypnosis, Acupuncture, Western herbal medicine and marigold therapy. The ASA has also ruled on complaints about claims for homeopathy made by the Society of Homeopaths, homeopath Steve Scrutton (and again) and the homeopathy lobby group Homeopathy: Medicine for the 21st Century.
We won ASA complaints last year over claims made by the Royal London Hospital for Integrated Medicine (RLHIM) about Medical and Clinical Hypnosis, Acupuncture, Western herbal medicine and marigold therapy. The ASA has also ruled on complaints about claims for homeopathy made by the Society of Homeopaths, homeopath Steve Scrutton (and again) and the homeopathy lobby group Homeopathy: Medicine for the 21st Century.
There's been the House of Commons Science and Technology Select Committee Evidence Check on homeopathy in 2010 that concluded:
11. In our view, the systematic reviews and meta-analyses conclusively demonstrate that homeopathic products perform no better than placebos.
The ASA also offer advertising advice about a wide range of alternative therapies and there are our other successful complaints to both the ASA and the medicines regulator, the MHRA.
So it comes as a surprise to find another two NHS hospitals making claims in a leaflet and on their websites for homeopathy, holistic approaches to cancer and depression, acupuncture, allergies and anthroposophic medicines (including mistletoe therapy for cancer).
The Glasgow Homeopathic Hospital (GHH) is one of just three hospitals left that are funded by the NHS — Tunbridge Wells Homeopathic Hospital closed in 2008 and the Homeopathic Hospital in Liverpool effectively disappeared a few years ago as well.
Part of NHS Greater Glasgow and Clyde, the GHH is located on the site of Gartnavel General Hospital. Like the Royal London Homeopathic Hospital, they are trying to re-brand themselves as the Centre for Integrative Care. It has itsown websitewhere you can take a tour of their very nice Healing Space (as they call it), opened in 1999 at atotal capital and building cost of £2,780,189 and costing the NHS over £2 million per annum in running costs.
The Bristol Homeopathic Hospital (BHH), part of University Hospitals Bristol NHS Foundation Trust, was recently downgraded from a city centre location to a clinic, now only sharing space in the South Bristol Community Hospital.
Decline
We've already seen the decline in homeopathy prescriptions on the NHS in England and Wales and this was examined further by Nancy K on her Evidence-Based Skepticism blog: Homeopathic harms vol. 8: Opportunity costs.
An FOIA request in 2011 by A Cuerden revealed some interesting figures for the GHH. The following charts show the number of new outpatient attendances, drawn from all over Scotland. There is certainly a decline as expected, but what is also interesting is to look at the number of return attendances — or rather the ratio of total attendances to new attendances. This is shown in the second chart, along with the trend.

The correlation coefficient between these two sets of data is -0.75. There could be several explanations for the increasing number of return attendances: one might be that their treatments are becoming less effective over time, requiring further sessions by patients, but other interpretations might spring to mind…
But it seems it's not just us who are pondering the future of the GHH: the building could be put to some good use as Scotland's first dedicated centre for chronic pain.
A similar FOIA request about the BHH tells us it costs around £350,000 per annum to run and gives some more interesting charts:

It's clear where they are headed.
Questionable claims
Getting back to the claims they were making, we were not convinced that the GHH or the BHH held the necessary evidence to substantiate them, so we submitted two complaints to the ASA: one about a GHH leafletwe obtained and a number of pages on their website and another complaint about claims on the BHH website.
The GHH say they provide a wide range of therapies: "Mindfulness Based Cognitive Therapy, Heartmath, Counselling, Art and Music Therapy, Physiotherapy, Therapeutic Massage, Allergy therapy and Anthroposophic medicine and complementary therapies such as Acupuncture, Homeopathy and Mistletoe Therapy." A few of these do have some good evidence behind them; others less so.
In their homeopathy leaflet, they stated:
out-patient homeopathic consultation
in out-patients, we see as full a range of conditions as a typical GP and are happy to treat any and multiple illnesses.
some examples of the problems we treat:
dermatology such as eczema, acne, psoriasis…
gynaecology such as pms, endometriosis, menopause…
gastroenterology such as IBS, IBD…
allergies at a specialist allergy clinic
childhood problems, such as behavioural difficulties, recurrent infections…
neurology, such as headaches, neuralgias, symptoms associated with MS…
psychiatry, such as anxiety, depression…
complementary cancer care, including Iscador
rheumatology, such as fibromyalgia, symptoms associated with, RA, OA…
cfs-me
We challenged all these claims, including their claim that they see "as full a range of conditions as a typical GP and are happy to treat any and multiple illnesses".
Our complaint about their website covered many claims made on their "Homepage", "Holistic Approach to Cancer", "Holistic Approach to Depression", "Acupuncture", "Allergy Service", "Anthroposophic Medicine", "Homeopathy" and "Mistletoe therapy" pages.
The ASA passed some of the points we made straight to their Compliance Team because they were clearly in breach of the ASA's guidance. They were going to fully investigate many of the other points we raised and asked the GHH for their response. However, that seems to have changed: they have now informally resolved the case with the ASA and have agreed to amend their website to comply with the ASA's guidance. The GHH is listed today on the ASA's website as one of their informally resolved cases, listed as NHS Greater Glasgow and Clyde.
We would have liked the ASA to have produced an adjudication so we could see how the GHH tried to substantiate their claims, particularly for mistletoe therapy, Heartmath, anthroposophic medicine as well as their more general claims. However, part of the ASA's job is to prevent the public from being misled and if it can do that by informally resolving complaints and having the claims withdrawn rather than by launching a full investigation, it usually means compliance is achieved more quickly. The end result is the same: misleading claims are removed.
Changes
The GHH have already made some minor changes to their website: their "Acupuncture", "Allergy Service" and "Anthroposophic Medicine" pages all changed on 01 April. We do not believe the pages are compliant yet and will continue to monitor them, so we may make further complaints to the ASA.
We'll let you know.
Bristol
The BHH claimed:
Homeopathy is useful in the management of:
- Rheumatology
- Allergic conditions
- Asthma
- Eczema and other dermatology conditions
- Menstrual and menopausal problems
- Digestive and bowel problems
- Stress and mood disorders
Because the ASA had already had a settled view on the evidence for homeopathy for these conditions, it was referred immediately to their Compliance Team to deal with. They haven't yet removed these from their website, but we'll leave it to the ASA to deal with that. We will, however, continue to monitor their website.
So, these are another two wins for us to add to the growing list — it is just unfortunate it took a complaint from us for these misleading claims to be removed.
HealthWatch help needed
 Our friends at the charity HealthWatch (they are in no way connected with any NHS Healthwatch body) ran a pilot study a few years ago on the effectiveness of consumer protection laws for regulating false claims of health benefits. They found that Trading Standards took very little decisive action, and avoided using the newest and most rigorous legislation.
Our friends at the charity HealthWatch (they are in no way connected with any NHS Healthwatch body) ran a pilot study a few years ago on the effectiveness of consumer protection laws for regulating false claims of health benefits. They found that Trading Standards took very little decisive action, and avoided using the newest and most rigorous legislation.
A much larger study is now being set up, and this will require a lot more help from volunteers. HealthWatch needs maybe 50 people to submit complaints to Trading Standards and to monitor progress over six months, using an online system.
If you would like to participate in this exciting project, please contact HealthWatch trustee This email address is being protected from spambots. You need JavaScript enabled to view it..
And finally...
Thanks to all who nominated and voted for us in the Skeptic magazine's Ockham Awards for Best Skeptic Campaign 2014. And, of course, thanks to Simon Singh for getting us set up and for his continuing support.
We were honoured to be presented with the award at the QED Conference in Manchester on 12 April by Robert Llewellyn, star of Red Dwarf and Scrapheap Challenge and evangelist for electric cars.
This was the third year running we had been nominated, but we managed to see off stiff competition from the other nominees: Guerrilla Skeptcism (US), the Houston Cancer Quack (US) and the Cosmic Genome (UK).
The award now takes pride of place on our bookshelf.
07 May 2014
The decline of homeopathy on the NHS
Homeopathy has long been provided on the National Health Service, but is it now in terminal decline?
Even though homeopathy is to some extent tolerated within the NHS and despite there being three homeopathic 'hospitals', it is clear that it is in decline. We know that these hospitals have been branching out into other areas and have even been re-branding themselves to move away from their reliance on homeopathy.
But just because it's been a part of the NHS since 1948 does not mean that homeopathy is endorsed by the NHS or the Government as being an effective treatment.
As the House of Commons Science and Technology Select Committee, after looking at the evidence and numerous submissions and after questioning scientists, homeopaths and others, stated in 2010:
In our view, the systematic reviews and meta-analyses conclusively demonstrate that homeopathic products perform no better than placebos.
And concluded:
The Government should stop allowing the funding of homeopathy on the NHS.
A very clear, concise and evidence-based conclusion. Unfortunately, the Government replied that it would leave it up to individual Primary Care Trusts (now effectively Clinical Commissioning Groups) to decide on the provision of homeopathy in their areas.
So how has homeopathy been faring?
We can get a good idea by looking at homeopathy prescriptions in the NHS in England.
Source data
Data on homeopathy prescriptions were obtained from Prescription Cost Analyses for England provided by the Health and Social Care Information Centre, with the help of a Freedom of Information Act request. These data may not show the total cost to the NHS as some items may be available via routes other than prescription. However, we believe they give a good indication of the number of prescriptions, the costs of these prescriptions and the average cost of a prescription.
Prescription Cost Analysis from 2004–2013
These data are published annually and can be found here. Homeopathic preparations are found under British National Formulary 19.2.3
Prescription Cost Analysis from 1998–2003
These data were published by the Department of Health and are available here.
Collectively, these data chart the decline in homeopathy on the NHS in England over the last 18 years:



We think these pictures speak a thousand words.
In the near future, we will be looking at where one particular homeopathic hospital gets a substantial chunk of its income from.
Update
03 April 2014
The data for 2013 were released today and the charts now include the new figures.
The downward trend of the last 17 years continues, with a further drop in the number of items prescribed of 15% from 2012 to 2013.
But the cost per item is still increasing, with inflation-busting price rises of 40%, 13% and 11% from 2010 and a further 15% increase from 2012 to 2013, giving a doubling of the cost per item since 2009.
The raw data for the charts can be downloaded here.
02 April 2014
Our published successes
A summary of our published complaint successes…so far
 We have had numerous successful complaints to the Advertising Standards Authority (ASA), the Medicines and Healthcare products Regulatory Agency (MHRA) and other regulators.
We have had numerous successful complaints to the Advertising Standards Authority (ASA), the Medicines and Healthcare products Regulatory Agency (MHRA) and other regulators.
New pages on our website detail these successes to date and we'll update them as we win further complaints.
Note that in all cases, it is the regulator that decides whether any complaint is valid or not and it is the regulator that assesses the complaint and decides the outcome according to the criteria laid down in their rules, regulations and laws.
We have no say in deciding these outcomes or the sanctions applied.
Note also that any decision an advertiser makes about the future of their business or how they choose to conduct it after any complaint is entirely a matter for them. All we seek is compliance with the appropriate rules, regulations and laws.
Further details of these complaints can usually be found in our News section.
We will update our results list as new complaints are published.
Advertising Standards Authority
The full list of ASA adjudications and informally resolved cases can he found here.
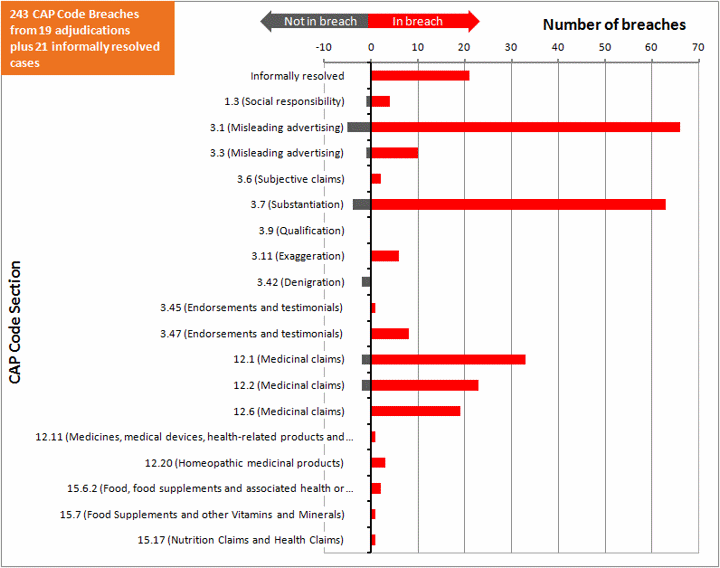
In summary:
- 15 upheld adjudications
- 4 partly upheld adjudications
- 18 informally resolved cases
- 85 issues investigated
- 231 instances of CAP Code breached identified
- 17 instances of CAP Code not breached identified
- 65 breaches of CAP Code 3.1 Misleading advertising
- 62 breaches of CAP Code 3.7 Substantiation (ie where the advertiser was unable to substantiate the claims made)
- 75 breaches of the CAP Codes on medical claims
The following chart shows the various sections of the CAP Code and the number of points found to be in breach and not in breach of these sections, as identified by the ASA. It also includes the number of informally resolved cases.
Medicines and Healthcare products Regulatory Agency
Five notices of complaints investigations have been published by the MHRA, covering some 29 sellers of homeopathy products. The full list can be found here.
Trading Standards
We have had several successes with Trading Standards but, unfortunately, outcomes are not published — unless a case ends up in court.
For example, we had successful complaints concerning a number of conferences giving advice on cancer treatments and high street Chinese herbalists making claims in their shop windows. We hope to bring you full details in the near future.
Complementary and Natural Healthcare Council
Our 100 complaints have been 'informally resolved' to the satisfaction of the CNHC, but they have published nothing about this. We will bring you more on this later.
Other regulators
We have had successes with the Health and Care Professions Council with complaints about 39 podiatrists advertising the unlicensed Marigold Therapy, but unfortunately, they have not published the outcome on their website.
22 February 2014
Bach Flower Remedies: foods, not medicines
Medicines regulator cancels product licences for non-medicines — now covered by food regulations
 They look like medicines: they have a licence number after all and come in a little glass bottle with a dropper and lots of detailed instructions, precautions, restrictions and warnings and even Boots, that trusted pharmacist on the high street, is in no doubt what they are:
They look like medicines: they have a licence number after all and come in a little glass bottle with a dropper and lots of detailed instructions, precautions, restrictions and warnings and even Boots, that trusted pharmacist on the high street, is in no doubt what they are:

There are a very precise 38 'remedies' in the set of original Bach Flower Remedies, all made from different flowers, invented in the 1930s by Dr Edward Bach (pronounced 'Batch'), a medical doctor who studied at University College Hospital, London. However, it wasn't his medical training that led him to come up with these flower remedies. He was also a homeopath, working for some time at the then Royal London Homeopathic Hospital, and apparently believed that:
…early morning sunlight passing through dew-drops on flower petals transferred the healing power of the flower onto the water.
Exactly what healing power of the flower he was referring to is not clear but the remedies were:
…intuitively derived and based on his perceived psychic connections to the plants
He gave them all their own little description, like this one for Pine:
Pine
You feel guilty or blame yourself.
“For those who blame themselves. Even when successful they think they could have done better, and are never satisfied with the decisions they make. Would this remedy help me to stop blaming myself for everything?”
These have been used as indications of what 'problem' each product is supposed to address.
Manufactured
Bach Flower Remedies are made by soaking flowers in water and exposing them to full sunlight for three hours or by boiling them in water (and left to cool, outdoors of course). They are then diluted in "40% proof" [sic] brandy, diluted further with grape alcohol and then bottled. The final product typically contains 27% by volume of alcohol.
The most well known one is, of course, Rescue Remedy® (a combination of five different flower remedies), used by many to calm their nerves in times of stress, or as the manufacturer, Nelsons, puts it:
…provide comfort and reassurance for daily stressful situations.
There are even kits available such as the Emotional Eating Kit — and where would they be without their celebrity endorsements?
There are now over 50 producers of flower remedies in the UK, but Nelsons is probably the most well-known one, selling products under the Bach Original Flower Remedies brand name with their trade marked![]() ®logo.
®logo.
The 'remedies' are divided into seven categories and have been given new names in recent years:
Old name → New name
Fear → Face your fears
Uncertainty → Know your own mind
Insufficient Interest in Present Circumstances → Live the day
Loneliness → Reach out to others
Oversensitivity to influences and ideas → Stand your ground
Despondency and Despair → Find joy and hope
The newer ones are even woollier than the old ones, but maybe the shift was to move them away from sounding too 'therapeutic'?
Bach finalised his set of 38 remedies in 1935. He died in 1936.
Do they work?
There is little doubt that someone who takes Flower Remedies may well believe they have an effect, but maybe there are placebo effects at play. What does the scientific evidence say?
There have been a few studies done:
A randomized, double-blind, placebo-controlled trial of a Bach Flower Remedy
It is concluded that ‘Five Flower Remedy’ had no specific effects in treating anxiety under these trial conditions.
We conclude that Bach-flower remedies are an effective placebo for test anxiety and do not have a specific effect.
Healing With Bach® Flower Essences: Testing a Complementary Therapy
The results suggest that BFE Rescue Remedy may be effective in reducing high levels of situational anxiety.
This last conclusion might come as a surprise, particularly since the trial was double-blinded, randomised and controlled. However, Prof Ernst has roundly criticised this trial as 'data dredging', saying that the positive result is "clearly based on a post hoc analysis".
It is clear there is no good reason to think Flower Remedies have any specific effects and therefore should not be considered medicines.
The Advertising Standards Authority are clear on what they would allow in advertising:
This therapy is described as a “therapeutic system that uses specially prepared plant infusions to balance physical and emotional disturbance”. Normally, flower ‘remedies’ are ingested to provide ‘energy’ to overcome negative thoughts. CAP is unaware of a relevant trade body or regulatory organisation. The method seems to lack scientific rigour and is supported mainly by anecdotal reports. In the absence of more compelling evidence, marketers are advised not to make claims for the efficacy of this treatment (Rule 12.1).
Legal status
We've mentioned Product Licences of Right (PLR) before in relation to homeopathic 'medicines', but the same applies to Bach Flower Remedies: they were given a free pass over 40 years ago and allocated a PLR licence number. Like homeopathy, the manufacturers have not had to provide any evidence whatsoever for claims made for these products. It is an anachronism that can only mislead the public.
The MHRA launched an informal consultation in January 2011 to look at some of these issues, hoping to use the upcoming review of the Medicines regulations to scrap the PLR scheme for all products:
The MHRA considers that it would be undesirable to use the current review of the Medicines Act and associated legislation to further perpetuate the existence of PLRs. This kind of licence, by its nature is envisaged as a pragmatic, temporary arrangement until products are reviewed and, where appropriate, moved to an ongoing regulatory scheme where they meet the relevant standards. It is highly desirable that product licensing schemes should reflect current regulatory standards and not represent a hangover provision from a number of decades ago. The review of the Medicines Act provides a suitable opportunity to bring the PLR arrangement to a close. This would also have the benefit for homeopathic products of achieving improved consistency of regulatory provision for labelling and advertising. This will better enable MHRA to regulate the market for these products. Improved patient information will benefit the consumer and facilitate informed choice.
In considering Flower Remedies specifically, they said:
A number of PLRs are for Bach flower remedies. MHRA intends to take the position, against the criteria set down in European legislation, that such products should normally no longer be regulated as medicines. Indeed there are many Bach flower remedies on the UK market, (and we understand on the markets of other EU Member States) that are legally supplied under other regulatory categories, such as food supplements. This change would represent a useful simplification and create a more level playing field for suppliers of this kind of product.
This didn't happen: there was no mention of this in the consolidated medicines regulations.
However, the MHRA have not been idle. There may well have been lobbying from Flower Remedy manufacturers — we suspect there was, but we don't yet know. But as a result of an FOIA request we submitted a few months ago, we now know that Bach Flower Remedies are no longer classed as medicines and have been relegated to being just food.
We asked the MHRA:
Question 4: Can you confirm that you still agree with your proposal of January 2011 that Bach flower remedies no longer be regulated as medicinal products? If so, what are the timescales for this?
Response 4: The MHRA contacted Nelsons on 29 July 2013 to advise them that all Product Licenses of Right for Bach flower remedies would be cancelled and that products quoting Product License of Right reference numbers and that include homeopathic/medicinal references on their packaging must be cleared from warehouses within 6 months and must not be put on the market after 28 January 2014. However, such products already on the market may be sold through and will not need to be recalled.
So, as of today, Bach Flower Remedies are not allowed to be placed on the market with a PLR licence number and they must have no medicinal or homeopathic references. That includes therapeutic indications, but exactly what that means isn't too clear.
We should now (or at least after products have cleared the supply chain) no longer see Bach Flower products with misleading licence numbers and they should no longer have homeopathic/medicinal references in their packaging or their advertising.
Just foodstuffs
Since they are no longer medicines, they are just foods now and health and nutrition claims fall within the remit of EU Directive 1924/2006, as enforced by European Food Standards Agency (EFSA). In the UK, advertising claims under EFSA regulations are regulated by the Advertising Standards Authority.
So, if advertisers of Bach Flower Remedies use the same claims as they have in the past, are they likely to be EFSA-compliant?
Given the lack of evidence, it would seem unlikely: but it's very easy to check the EFSA Register on nutrition and health claims. The Bach Flower products are, of course, mostly alcohol, but even if the decoctions of the various flowers are in sufficient quantities to be considered ingredients, it would be easy to check the EFSA register for all 38.
But there is no need.
Foodstuffs that contain more than 1.2% by volume of alcohol are singled out specifically in the EFSA rules:
Beverages containing more than 1,2 % by volume of alcohol shall not bear:
(a) health claims;
(b) nutrition claims, other than those which refer to a reduction in the alcohol or energy content.
So, because Flower Remedies contain little more than alcohol, they are not allowed any health claims whatsoever. Whether the flower ingredients themselves warranted any authorised health claims is entirely moot.
But are the words in the category names implied health claims? Is the woolly description for each product a health claim? Can they still be called 'remedies'? What about 'Rescue Remedy'?
A German court gives us the answer.
In court
In Germany, there is no equivalent PLR scheme and in August 2013, a regional court in Bielefeld, Germany confirmed that Bach Flower products are indeed covered by the EFSA regulations (HCVO in German) and confirmed that medicinal claims were not allowed.
This was a case brought by a trade association against a pharmacist who was selling Bach Flower products online. The association claimed unfair competitor behaviour from the pharmacist by falsely advertising both "RESCUE® - The original Bach® Flower mix" and "original Bach Flower essences" in general with health claims. Note the slightly different product names used in Germany — they don't include the word 'remedy', although this is sometimes seen on Bach products in Germany directly imported from abroad.
The defending pharmacist was supported in their case by their supplier for these products, that in the court's judgement was referred to as "the German subsidiary of B. & Co. Ltd. from England", as "sister company of B. Ltd. - the producer of the original Bach Flower products", and as "the German sales branch for all their respective products". We believe this means that they were supported by Nelson GmbH in Hamburg, the German distribution subsidiary of A Nelson & Co Limited, the multi-million pound UK company that produces Nelsons homeopathic products.
The accused argued that they didn't make any actual health claims but only referred to "potential improvements in general well-being". The court wouldn't have any of this, as they said the advertisement clearly refers to specific circumstances of life where the Flower products "could be" helpful, and the HCVO regulation asks for a broad interpretation of "health claims" to make sure consumers are protected: "even unspecific indications with reference to health are to be considered health-related indications under the HCVO".
When the supplier argued to support the accused, they suggested their ordinary customer would not consider their claims as health-specific (which would require scientific evidence), but only expect that "those products were designed for and would have some impact on specific everyday emotional states". "These expectations are fulfilled by the Bach Flower products", they continued, "be it because of their energetic properties as ascribed to them by Edward Bach, or because of the reminder or suggestive function that accompanies their consumption." They would appear to be simply saying the products provide placebo effects and no more!
But the court ruled these were claims towards psychological support and hence qualified as health claims no matter how unspecific the manufacturer thinks the claims were.
So it does appear that even the woolly claims are not allowed, and the pharmacist was told to stop making them. If the pharmacist continues to make the stated claims, they can fine him up to €250,000 cash or send him to jail for up to two years if he doesn't pay the fine. That's worth repeating: up to €250,000 in fines or up to two years in jail.
The pharmacist has to pay for the trial costs, and the Bach Flower company, who supported him in his defence, has to cover their own costs.
How much this has cost the pharmacist and the German and UK Bach Flower companies we cannot know. Because of the importance of the case, we suspect it was fought hard. What we can do is look at the finances of the UK company:
 Data from Company Check
Data from Company Check
The future
It's good to see the MHRA finally catch up and cancel the PLRs for these non-medicines — even if it has taken 40 years — and we urge them to do the same for homeopathy PLR products so that the public are not misled into thinking they are medicines.
However, now Bach Flower products are just foods, we look forward to manufacturers and all others who supply and advertise these products to fully comply with the EFSA regulations, enabling the public to make fully informed choices.
We believe the German ruling sets a precedent that is binding in the UK, but if we have to test the various claims for Bach Flower Remedies with a complaint to the ASA, we will.
Acknowledgements
Thanks to Sven Rudloff for help in understanding the German ruling and Aribert Deckers for bringing it to our attention in the first place.
29 January 2014
Update
02 February 2014
The Advertising Standards Authority has now updated its guidance on Therapies: Bach and other flower remedies:
This section should be read in conjunction with the entry on ‘Therapies: General’.
Bach flower remedies are described as “a system of 38 Flower Remedies to help mankind achieve joy and happiness”. CAP understands that at the time the Medicines Act (1971) was implemented, Product Licences of Right (PLRs) were issued to all medicines, including homeopathic remedies, and that a number of PLRs were granted for Bach flower remedies.
In January 2014 the MHRA took the decision that Bach flower remedies would no longer be regulated as medicines but instead be classified as foods. Any health or nutrition claims made for foods must be made in accordance with those claims permitted on the EU Register and Annex. CAP understands that some Bach flower remedies contain levels of alcohol which would preclude them from bearing health claims altogether (Rule 18.17). While it may be possible for a flower remedy to carry a nutrition claim, the nutrition claims permitted for products containing alcohol are limited.
The end of Product Licences of Right?
 The (virtual) ink on our last newsletter had barely dried before a new document was published by the MHRA
The (virtual) ink on our last newsletter had barely dried before a new document was published by the MHRA
Last Friday, the medicines regulator (the MHRA), signaled its desire to finally end the 40-year anachronism of homeopathic Product Licences of Right (PLRs):
A mouthful of a title, but its aim is clear: to encourage homeopathy manufacturers to transfer their PLR products to either the Simplified Homeopathic Rules (HR) Scheme or the National Rules (NR) scheme.
For the past 42 years, these products have been given a free ride in terms of not having to comply with the same rules as other products classed as medicines. As the MHRA says:
Product Licences of Right (PLRs) were issued to all medicinal products on the market at the time that the Medicines Act 1968 was implemented (in 1971). PLRs were envisaged as a temporary arrangement until products were reviewed and, where appropriate, moved to an ongoing regulatory scheme where they would meet the relevant standards. Most categories of medicine, except homeopathic medicines, which were exempted under Directive 75/319EEC, were subsequently reviewed by the early 1990’s and products were either granted a full Product Licence or the PLR was revoked.
Their announcement said:
Product Licences of Right (PLRs) holders are invited to transfer certain homeopathic products with PLRs to either homeopathic marketing authorisations under the National Rules Scheme or homeopathic registration certificates under the Simplified Registration Scheme.
Unfortunately, the MHRA has not given a deadline, and the MHRA say they will transfer for free (for the time being at least), so that the manufacturers will not even have the burden of the usual fees they would have to pay for HR or NR applications.

According to the MHRA's data (obtained through a Freedom of Information Request), there are 406 PLR products, most of which are homeopathic. These include 32 New Era products (that Seven Seas/Merck abandoned earlier this year), 319 Weleda, 54 Nelsons and one product from the (possibly now defunct) Anglo German Homeopathic Centre, based in Hereford.
In many ways this is good move: any PLR products that transfer to the HR scheme will not be allowed indications (ie to say what medical conditions they can be used for) and will all have to have the following text on the product:
Homeopathic medicinal product without approved therapeutic indications
Products that are transferred to the NR scheme will be allowed indications if the manufacturer can provide 'evidence' for therapeutic use for that indication. Of course, no actual evidence at all is required: they just have to show that it’s been used for whatever the condition by homeopaths in the past or provide a 'proving' for it.
However, this means they are brought under control to some extent and are more tightly controlled in terms of advertising. At least NR products have to carry the wording:
A homeopathic medicinal product used within the homeopathic tradition for the relief of or treatment of…
…followed by the conditions it can ‘treat’ as permitted by the product’s Public Assessment Report.
Teething troubles
Take Nelsons Teetha Teething Gel that was authorised last year (not to be confused with Nelsons PLR Teetha teething granules, pictured above). This has the official-sounding NR authorisation number NR 01175/0184 and has the 'active ingredients' Chamomilla recutica, Aconitum napellus and Atropa belladonna. Although these may sound dangerous — particularly the belladonna — these are all at 12C dilution, so, as long as they are properly and carefully diluted, under an adequate quality regime, there is just a 60% probability of it containing just one molecule of the original material if one mole of the original substance was used. This product's Public Assessment Report (PAR) give details of the product and its assessment.
Under the heading Evidence supporting the proposed indication, it states:
No new clinical data were submitted and none are required for an application of this type. In support of this application details of homeopathic provings and published scientific literature have been provided. These are adequate evidence to support the indications for which a national rules authorisation is sought.
And what did this 'evidence' substantiate in terms of indications?
A homeopathic medicinal product used within the homeopathic tradition for the symptomatic relief of teething pain and the symptoms associated with teething which are sore and tender gums, flushed cheeks and dribbling.
Anyway, at least this limits what therapeutic claims can be made, even if they are not based on any measure of credible evidence. The same will apply to any PLR products transferred to the NR scheme.
 Meeting the standards
Meeting the standards
There are, of course, manufacturing standards to be met even for the HR scheme. Providing the dossier required by the MHRA for an application should be relatively trivial for any competent manufacturer, but some may well struggle. The contents of the dossier required for submission is given here: Homeopathic applications (Simplified Registration Scheme and the National Rules Scheme).
There are ongoing periodic fees, but the periodic fee for a NR product is the same as that for a PLR; this measly £80 is only a tiny fraction of the fee required by the MHRA for 'Big Pharma' medicines and the periodic fee for an HR scheme product is zero.
In the right direction
Allowing any homeopathic product to make therapeutic claims is hardly ideal and unwitting members of the public could still be misled into thinking these homeopathic products are real medicines, but at least this a step in the right direction for consumer protection.
At present, the MHRA have not said when they would like all PLRs to be either ended or withdrawn, but we urge them to scrap PLRs completely as soon as possible. There is no reason why this anachronism should be allowed to continue any longer and we hope that PLR products will finally be consigned to history.
Provings
A final word about 'provings': these are used to 'justify' the indications that are permitted for National Rules Scheme products.
However, the word is derived from the German Prüfung, meaning 'a test', and does not mean they provide any kind of proof that the product is effective for these indications. In the words of the Society of Homeopaths:
A pre-defined number of repeated doses of the homeopathic remedy are given to healthy volunteers until symptoms are experienced. These are collated by observers and distinctive symptoms common to multiple participants (which are most likely to be related to the medicine) are identified. According to the central homeopathic principle that ‘like cures like’, the remedy may have the potential to treat these specific characteristic symptoms.
Most readers will be able to spot rather obvious flaws in this thinking. They also like to call them 'homeopathic pathogenetic trials', but they are not clinical trials in the conventional sense or indeed in any meaningful sense.
We have more to say about provings and their status…but you'll need to wait for our next newsletter to find out!
10 November 2013
Obeying the rules
 Since we started, we've been quietly submitting complaints to the medicines regulator about sellers of homeopathy 'medicines' who appeared to be not complying with various medicines regulations.
Since we started, we've been quietly submitting complaints to the medicines regulator about sellers of homeopathy 'medicines' who appeared to be not complying with various medicines regulations.
It's time to let you know about our 30 successes.
There are rules surrounding the advertising of medicines. There have to be to protect the public from misleading claims. It's illegal in the UK, for example, to advertise prescription medicines to the public. That protects the public being swayed by advertising gloss, spin and downright deception and probably saves many a GP from being bombarded by requests for medicines they do not need.
But there are also rules and regulations surrounding homeopathic medicines. These are enshrined in various EU Directives and transposed into UK laws and regulations. (Unfortunately, those same Directives call them 'medicines' — this is highly misleading in itself, of course, but, when discussing the regulations formally, it is easier to stick to the terms it uses.)
All medicines in the UK are regulated by the Medicines and Healthcare products Regulatory Agency (MHRA) and they publish their guidance on advertising in what they call their Blue Guide: Advertising and Promotion of Medicines in the UK. A special Annex gives guidance on the advertising of homeopathic medicines that also gives useful information on the underlying EU Declarations and legislation.
But first, some background on the categories of homeopathic products that the MHRA 'regulate':
- Marketing Authorisation (MA)
- Product Licences of Right (PLR)
- National Rules Scheme (NR)
- Simplified Scheme or Homeopathic Registration (HR)
There are no homeopathic medicines that have Market Authorisation because evidence of efficacy is required — a dizzy height homeopathy has never been able to reach, of course. Conventional medicines have MAs.
Product License of Right (PLR)
There are under 500 homeopathic products that have Product Licences of Right, but the only requirement they had to meet to gain this licence was that they were on sale in 1971 when the Medicines Act 1968 came into force: no proof of safety; no proof of efficacy. In fact, all medicines, homeopathic or not, were automatically given PLRs by the MHRA’s predecessor, the Medicines Control Agency. But, by the early 1990s, most conventional medicines had been reviewed and either given a full product licence (MA) if they met the criteria (including evidence for efficacy) or they were withdrawn from the market.
The MHRA allowed homeopathic medicines, Bach flower 'remedies' and Anthroposophic 'medicines' to remain with PLRs and continue to make claims about what medical conditions they can be used for. The MHRA was minded to remove this category a few years ago, but has unfortunately backtracked on that. One possible reason for this might have been pressure from the homeopathy industry, wanting to continue to freely make unevidenced claims about their products. We hope to say more about this and PLRs in a future newsletter.
National Rules Scheme (NR)
The next category is the National Rules Scheme. Homeopathic medicines authorised in this category are permitted to say they can be used for the 'relief or treatment of minor symptoms or minor conditions in humans' — those that 'can ordinarily and with reasonable safety be relieved or treated without the supervision or intervention of a doctor'. In the jargon, they are allowed to claim 'therapeutic indications', but only those that have been permitted by the MHRA for each individual product as stated in their Public Assessment Report (eg this for Nelson's Teetha teething gel). For various reasons, there are just eight homeopathic medicines in this category, with most of them appearing in just this last year, despite the scheme being launched in 2006. The mandatory wording on the product is:
A homeopathic medicinal product used within the homeopathic tradition for the relief of or treatment of…
…followed by the permitted indications. Note that these indications come from historic homeopathic literature of what it's been used to treat or from 'provings'. These 'provings' (from the German Prüfung, meaning a test, not a proof), although called 'homeopathic pathogenetic trials' by some homeopaths, are not clinical trials in the conventional sense — but this yet another topic for a future newsletter.
Homeopathic Rules Scheme (HR)
The final category is Homeopathic Registration: the MHRA has issued fewer than 300 registrations to ten different homeopathy manufacturers. No evidence of efficacy is required, of course, but they are not allowed to make any therapeutic claims either — how could a regulated product be allowed to make claims when it's not been tested for those claims? Instead, they must have the following words on them:
A homeopathic medicinal product without approved therapeutic indications.
Whether or not an unsuspecting member of the public understands what this jargon means is a moot point.
A full list of all NR and HR homeopathic medicines is available from the MHRA.
Any homeopathic medicine not falling into one of the above categories is an unlicensed medicine. As such, there are strict rules on advertising them: it is illegal to advertise an unlicensed medicine to the public.
The double-standards and the extremely low hurdle to be achieved by homeopathic products should be obvious.
In summary, for homeopathic products:
- PLR homeopathic medicines can advertise with indications;
- NR homeopathic medicines can advertise those indications that have been authorised;
- HR homeopathic medicines cannot be associated with any indications;
- Unlicensed medicines cannot be advertised to the public.
However, we have found may sellers of homeopathic medicines advertising outside these restrictions. We've highlighted a few in the past, mainly Holland and Barrett, but we have been complaining about others too.
Because these have taken time to be resolved and have been resolved at different times, we've said little before now. However, now is the time to publicise our successes.
Boiron's Oscillococcinum
Several UK sellers were advertising Boiron's Oscillococcinum. This is an unlicensed medicine in the UK. The sellers included:
- Metro Health & Beauty Ltd.
- Baby Clothes & More!
- John Bell & Croyden
- Life Without Drugs
- Price Grabber
- Victoria Health
 …and the following amazon.co.uk and/or ebay.co.uk sellers:
…and the following amazon.co.uk and/or ebay.co.uk sellers:
While many of these have been removed after being contacted by the MHRA, there are a few others, including several registered pharmacies, that have yet to make the necessary changes. Further action is being taken and we'll let you know the outcome in due course. Some of these complaints about registered pharmacies were passed to the statutory pharmacy regulator, the General Pharmaceutical Council. However, there are further issues they have yet to deal with.
However, the MHRA were unable to take action against one seller, Doc Simon, because, although they have a UK domain name and look to all intents and purposes like a UK company, they appear to be based in the Czech Republic. It is not illegal to import unlicensed medicines into the UK for your own personal use, so there was nothing the MHRA could do about this website, even though they are advertising and selling to the UK.
Unfortunately, the MHRA have not published the outcomes of these complaints, so it's been left to us to make people aware of them.
We will continue to monitor all those websites, of course.
Main homeopathy manufacturers
Some of the major homeopathy manufacturers have already had to make changes to their websites after we submitted complaints to the MHRA, including Nelsons. We currently have several other complaints running — these are proving more time-consuming to resolve, but progress is being made and we will let you know when these are concluded.
 Other sellers of homeopathy
Other sellers of homeopathy
We've submitted complaints to the MHRA about many other advertisers of homeopathic medicines, including one ebay seller, Wingate, where we questioned several aspects of their advertising, including incorrect labelling of re-branded products. Again, the MHRA chose not to publish the outcome of these complaints on their website.
However, the outcomes of our latest batch of complaints to the MHRA were recently published.
As we've seen, the MHRA do not always announce the results of their investigations, so we welcome this publication that stands as a marker for other sellers that they have to check that their advertising is compliant.
The common issues we found were:
- Advertising HR registered products with indications
- Advertising NR products with indications not permitted by the product's PAR
- Advertising unlicensed medicines;
- Advertising of kits of homeopathic medicines not authorised by the MHRA.
The sellers we complained about included:
- Apothecary Shop
- Buxton & Grant Pharmacy
- Chemist Direct
- Crossgates Farm
- Dr Reckeweg
- Elixir Health
- Garden Pharmacy
- Healthy Route
- Holland & Barrett (further point-of-sale advertising issues with Nelsons homeopathic products)
- Homeopath.it (although this appears to be an Italian domain, it redirects to www.homeopathshop.co.uk)
- HSC Online
- Jan de Vries Healthcare
- Little Birth Kit Company
- Natural Practices Clinic
- Organic Pharmacy
- Pure Health Clinic (this was not one of our complaints, so we have no idea why the MHRA gave us credit for it)
- Traumeel Remedy
- Woodland Herbs
We have since checked these websites and have notified the MHRA that we believe some of them are still in breach of the advertising regulations. Others are no longer advertising any homeopathic products.
We believe this is good progress and we hope the MHRA will publish the outcomes of future complaints.
However, the MHRA is still dealing with some seven others, including some Registered Pharmacies and homeopathy manufacturer. Once these have been concluded, we'll publicise the results.
Out of this last batch, there are a couple of particular interest.
Dr Reckeweg
This website was one of the main sellers of a homeopathic product called Schüssler (Schuessler) Tissue Salts. These have been touted as the replacement to New Era — owned by Seven Seas, who in turn are owned by pharmaceutical giant Merck — tissue salts (which had a PLR) that were withdrawn earlier this year. The New Era brand seems to have been sold to Olimed Limited, a subsidiary of Italian company Named SpA, so we can probably expect to see these products re-appear in the future.
However, Schüssler products are not registered or authorised and are therefore unlicensed medicines. Many other retailers have been selling them, but this appears to be their main website in the UK. This shop now displays the message:
 Traumeel Remedy
Traumeel Remedy
This website was selling products manufactured by Heel GmbH, but the shop facility closed after our complaint. This range includes all sorts of homeopathic products, some of which are injectable. As these are neither registered nor authorised by the MHRA, they are unlicensed medicines.
After our initial complaint, the owner, Sarah Bell, sent out an email to her mailing list (all typos as in the original):
Recently I have been hassled by the never ending growing body of rules and regulations which are set out here in the UK regarding homeopathic remedies and the Heel remedies. It is a vicious monster in my opinion and does a great deal of harm.
Fortunately we have set up a system which enables me to contiue to supply and help you obtain the Heel remedies. So, do not worry. I have your back.
Although I now understand why many practitioners in the UK baulk at the prospect as it is difficult and requries stamina to say the least. Thick skin?! I think so!
However this is my problem not yours.
I will continue to provide access to you of the complete Heel range of remedies. I may in future be updating the blog in the private shop site and not the public site in order to comply with their regulations.
I get very annoyed as due to their regulations they make it extremely difficult and expensive for most people to obtain what I think is an outstanding range of homeopthic remedies which go such a long way to helping people maintain phenomenal health - so how dare they do everything they can to make them extremely difficult to buy and obtain?
C'est la vie - we are in a society run by so many rules that they counteract all the good they are supposed to do.
The quote I read was:-
Every adversity, every failure, every heartache carries with it the seed of an equal or greater benefit. - Napolen Hill
Do you have any inspiring quotes that help you get through tough situations? If you do, just reply to this email! I'd love to hear what helps you when times are tough. I think of it as fighting an ongoing battle - you and I together.
We will live on another day and keep on fighting this battle!
And finally my "call to arms" - please pass on the private shop link to friends and family and colleagues. Help me continue to keep helping those who know drugs are not the answer.
http://shop.traumeelremedy.com
Lastly, to quote a great man...
"Never, ever, ever, ever, ever, ever, ever, give in. Never give in. Never give in. Never give in." - Winston Churchill (but you already knew that!)
She even Tweeted about her new 'private' shop:
Struggling with Bronchitis & chest infections? Contact me - I will send you a private shop link so you can buy Heel Homeopathic Remedies
So, instead of simply obeying the statutory medicines regulator, Bell opened up a new 'private' shop where she could carry on selling the unlicensed homeopathic medicines to her customers. We passed her email on to the MHRA; the shop facility no longer exists.
However, the website still contains an advert for Heel products and a contact form…
Although she is the main seller in the UK, Heel GmbH homeopathic products are still available from some other suppliers, so we’ve pointed these out to the MHRA.
Obeying the rules
As we have explained above, the regulation surrounding the advertising of homeopathic 'medicines' are fairly straightforward and easy to understand. They are not arbitrary and complying with them is not optional: they are legal requirements. That so many homeopathy traders and advertisers have been found to be non-compliant suggests a wide-spread disregard for the medicines regulations, which are there to protect the public from misleading, false and unsubstantiated claims.
All homeopathy manufacturers, advertisers and sellers have an ethical and legal obligation to abide by them — whether they like them or not.
01 November 2013
Latest news
- "Undisputable evidence of scientific misconduct" by homeopaths
- Yet another bad year for homeopathy
- Nelsons Homeopathic Pharmacy #3
- Nelsons Homeopathic Pharmacy #2
- The Society of Homeopaths: failing to make the case for homeopathy
- The end of homeopathy on the NHS in Bristol?
- NHS Homeopathy: 20 years of decline
- The different faces of the Society of Homeopaths
- The growing pains of osteopaths
- Diluting misleading claims - ASA update
Most read
- Finding deleted and changed webpages
- About The Nightingale Collaboration
- How to find out who owns a website
- Advertising Standards Authority
- Rubbing salts into the wounds of homeopathy
- How to submit a complaint to the ASA
- The decline of homeopathy on the NHS
- Landmark decisions for homeopaths
- NHS Lanarkshire to end referrals to Glasgow Homeopathic Hospital
- Making a complaint